24-7
√ 100% SAFE

You’re always SAFE with OptiMate, all OptiMate chargers have a 24-7 battery maintenance program designed to prolong the life and maximize the strength of a stored battery. All you need to do is to make sure your OptiMate charger is suited for your battery type.

Just connect the charger to the battery and walk away, the battery can be safely left connected for months or years as the program adjusts according to the battery’s needs.
The advanced 24/7 battery maintenance program periodically delivers just the right amount to keep the battery optimally charged, but never overcharging. This process will continue for as long as you keep your OptiMate connected to the battery.
√ All Starter & Deep Cycle batteries

All Starter & Deep Cycle batteries need maintenance. The need for battery maintenance mostly depends on these two variables:
1) Battery type: the fact that you have a Lead-acid, or a Lithium-Ion battery will determine what maintenance is best to always insure top level performance.
2) How fast the battery loses charge due to self-discharge and/or the daily needs of your vehicle’s electronics system (parasitic draw)
1) Battery type:
Lead-acid & Lithium-Ion batteries differ in how they generate electricity and therefore need different maintenance charging when stored. Scroll down the page to find out more on these processes.
2) How fast charge is lost depends on the self-discharge rate of your starter battery and the parasitic draw.
a. Energy loss due to self-discharge: it is normal for a battery to lose charge when inactive. Stored outside a vehicle, a Lead-acid battery will self-discharge much faster than a Lithium-Ion battery.
b. Energy loss due to the parasitic draw: your vehicle’s electronics system and all its memorized settings (radio stations in your car, suspension settings, ride mode setting on your motorcycle etc..) requires energy from your starter battery to function, this energy needed daily is called the parasitic draw.
Lead-acid (Pb) – normal process
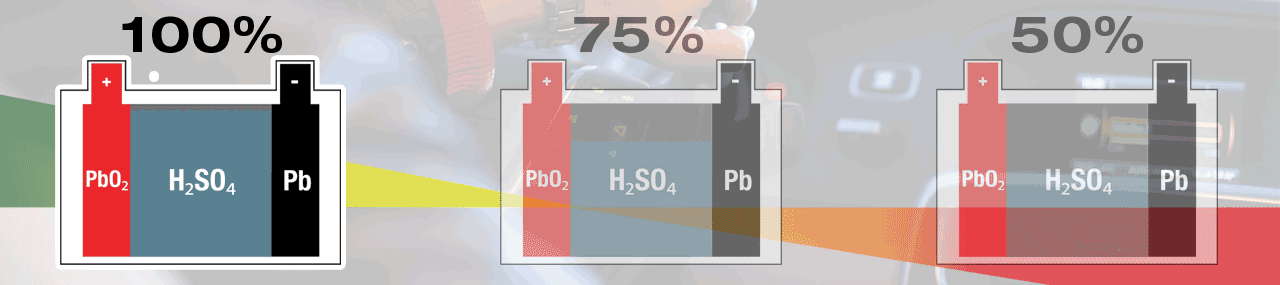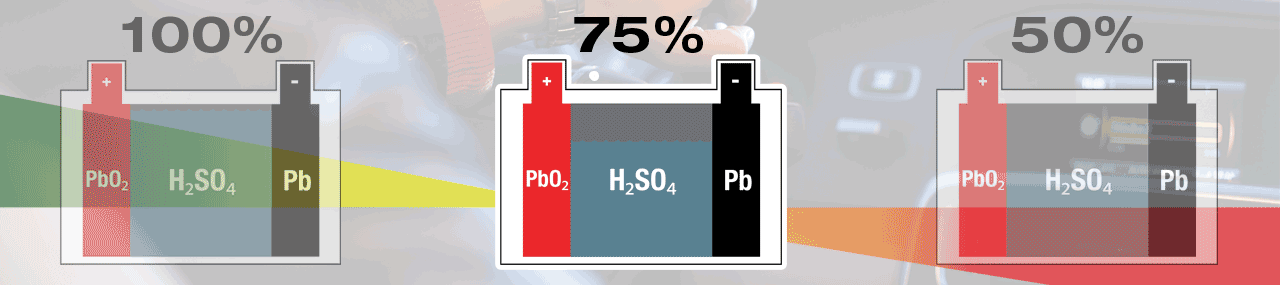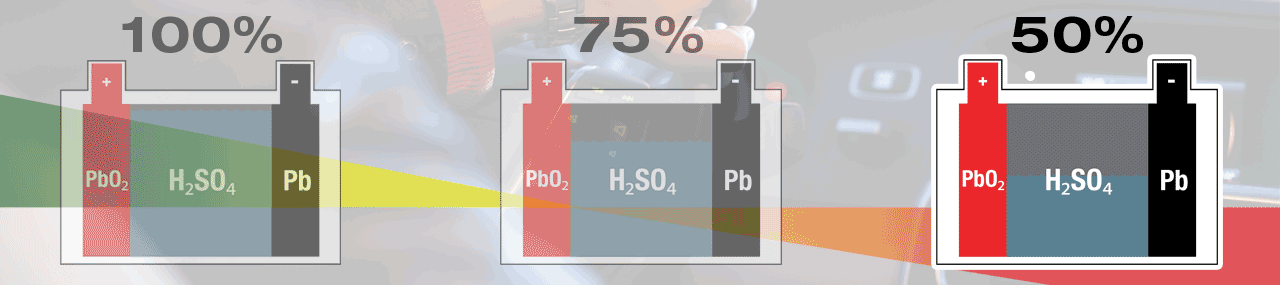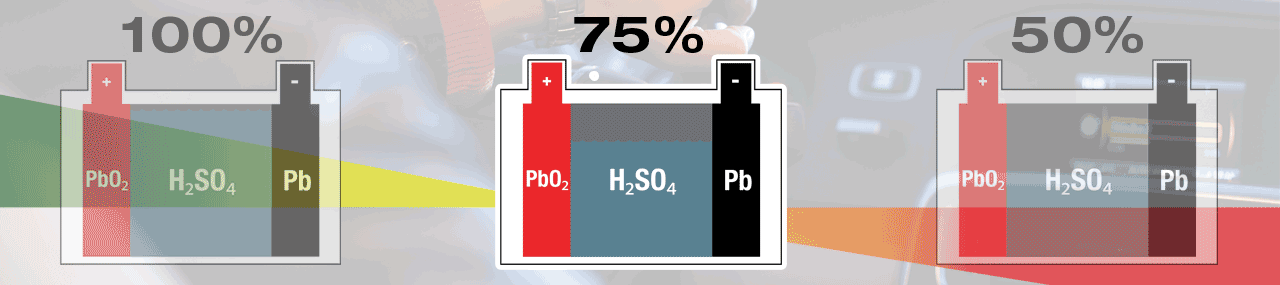
A battery’s normal behaviour during a start-cycle is to self-discharge to give energy to start your engine. Once the engine is turned over – running, the vehicle’s charge system will give energy to the battery and bring it back to full (100%) charge. The lead-sulphate created during this discharge process will be recycled soon after, and in your fully charged (100%) battery, no lead-sulphate is present.
To check the vehicle’s charge system, Optimate has a range of Battery Testers that can tell you, on the spot, if your system and your battery are performing as they should.
Lead-acid (Pb) – abnormal process
When the battery is not used or re-charged, lead-sulphate sticks to your battery poles (+ and -) and hardens or crystalizes. The more crystalized lead-sulphate in your battery, the weaker it becomes. Not using your battery or not charging your battery enough creates a deeply discharged – DEAD battery.
Lead-acid (Pb) – solution

The Solution is Optimate, our SILVER or GOLD series chargers can solve this problem for you. Connect one of these chargers to your DEAD battery, and a SAVE program will initiate that will safely bring your battery back to full charge. This process will recycle your hardened waste chemical (Lead-Sulphate) from your battery poles and re-activate the sulphuric acid.
If your battery is too weak to start over your engine, always disconnect it from your vehicle’s electronics before you connect the SILVER or GOLD charger. This way, the charger can safely use all its desulphating powers on your battery. Leaving the battery connected in your vehicle when you connect the SILVER or GOLD charger, limits the power of the charger to insure no damage is created onto your vehicle’s electronics, but this limitation can cause the problem on the battery to remain.
Lead-acid (Pb) – self-discharge
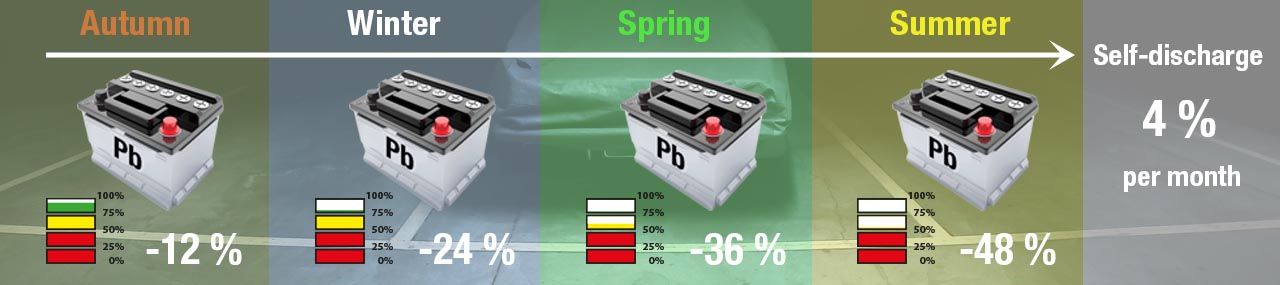
All batteries self-discharge when stored, even if not connected to electrical circuitry, using their own stored energy to maintain their voltage. A lead-acid battery discharges at 4% per month. If your vehicle is stored the whole winter for example, your lead acid battery will have lost 12% charge due to self-discharge.
√ 100% happy Pb battery

Lead-acid batteries require maintenance charging more frequently, to ensure that all ‘waste’ chemicals created during charging, are promptly and completely recycled. The most urgent is the ‘waste’ chemical lead-sulphate (PbSO4), it will crystallize if left dormant for too long, even when the battery voltage still appears to be sufficient. (Learn more here – Pb). OptiMate’s 24-7 maintenance program checks charge level hourly and delivers sufficient charge to keep the battery at 100%, with a continuous ‘desulphation’ pulse breaking up any remaining crystallized lead-sulphate. A lead-acid battery kept at full charge remains strong for longer.
Lithium-ion (Li-ion) – normal process

A Lithium-Ion battery’s normal behaviour during a start-cycle is to self-discharge to give energy to start your engine. Once the engine has started and is running, the vehicle’s charge system will give energy back to the battery and bring it up to full (100%) charge.
A Lithium battery passively generates electricity; Li-Ions flow from the anode (-) to the cathode (+) of a cell and that causes static electricity. When receiving charge the Li-ion flow is reversed, Li-Ions are returned to the anode. It is essential that a balance is maintained and there are sufficient Li-Ions at the anode and cathode after discharge or recharge.
Lithium-ion (Li-ion) – abnormal process
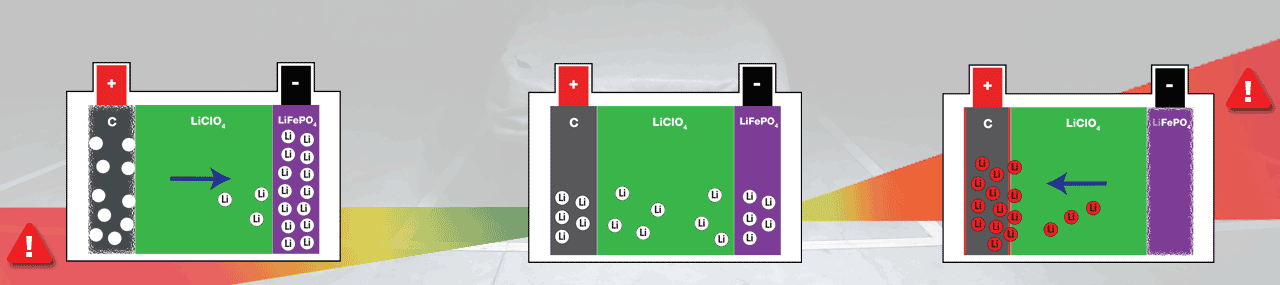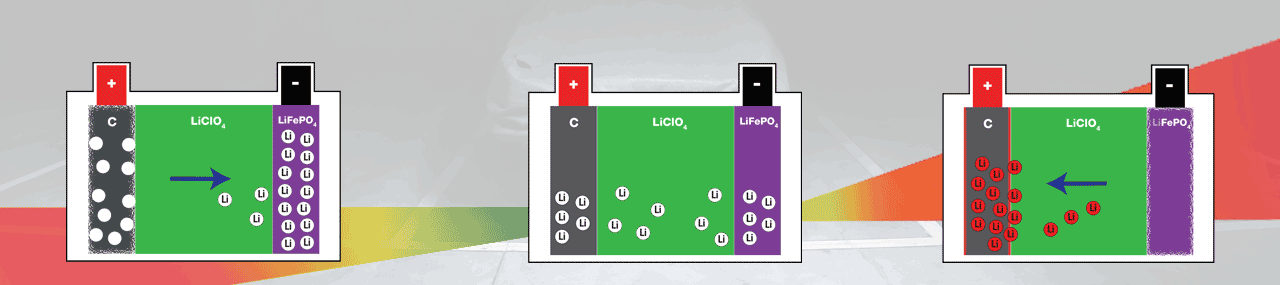
Abnormal behaviour for a Lithium-Ion battery is when it gets over discharged, or when it over charges (danger zones on the graphics). You can prevent both by using an OptiMate charger suited for Lithium-ion batteries.
All Li-Ions will have migrated to the Anode (-), leaving the cathode (+) ‘empty’ and fragile.
All Li-ions will have migrated to the cathode (+), leaving the anode (-) ‘empty’ and fragile.
Lithium-ion (Li-ion) – solution

Both over-discharge and -charge can cause permanent damage to the battery. Connect it to an OptiMate Lithium series charger to solve and/or prevent issues on your lithium-ion battery.
Lithium-ion (Li-ion) – self-discharge

All batteries self-discharge when stored, even if not connected to electrical circuitry, using their own stored energy to maintain their voltage. A Lithium battery discharges at 1% per month. Leave your Lithium battery uncharged for the winter, and it will have only lost about 3% of its charge.
Discharge when connected to a vehicle’s electronics can affect discharge speed due to the parasitic draw.
√ 100% happy Li-ion battery

Lithium batteries have the easiest maintenance routine, the battery remains healthy if kept above its minimum recommended voltage / State Of Charge. It might only need charge occasionally.
OptiMate 24-7 battery maintenance program checks charge level hourly and will deliver charge when necessary, keeping the battery within a 70-90% state of charge. A Lithium battery kept at the right charge remains strong for longer.
√ Lead-acid (Pb) vs. Lithium-ion (Li-ion)
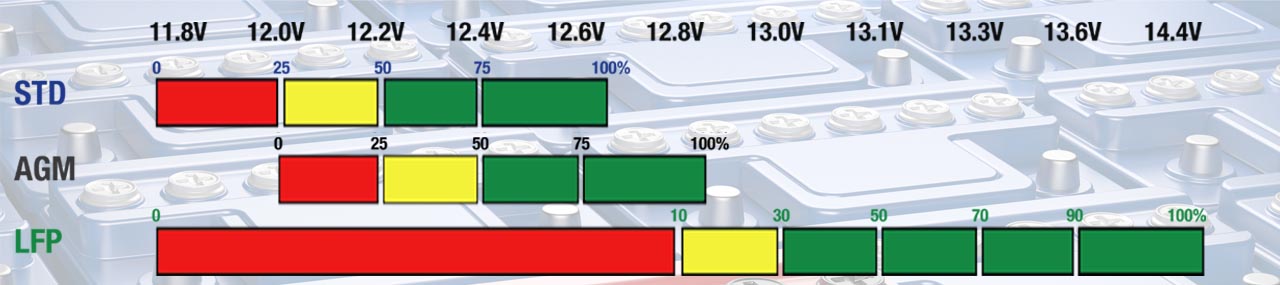
Deep discharge below the battery’s safe voltage level weakens any battery. Even if recovered, the battery is not as strong as it used to be, subsequently the battery works harder which causes more stress, resulting in a shortened lifespan.
Parasitic draw
Parasitic current draw from connected circuitry (such as ‘always on’ electronics in a stored vehicle) add to the battery’s self-discharge current; the battery discharges faster. The storage capacity (in Ah) of the battery determines how long the battery remains within its safe operating range, a smaller battery with a lower Ah rating will reach its minimum safe voltage level quicker. As an example, a typical 10 milli-amp draw from connected circuitry causes loss of 1.7 Ah every 7 days.
FAQ
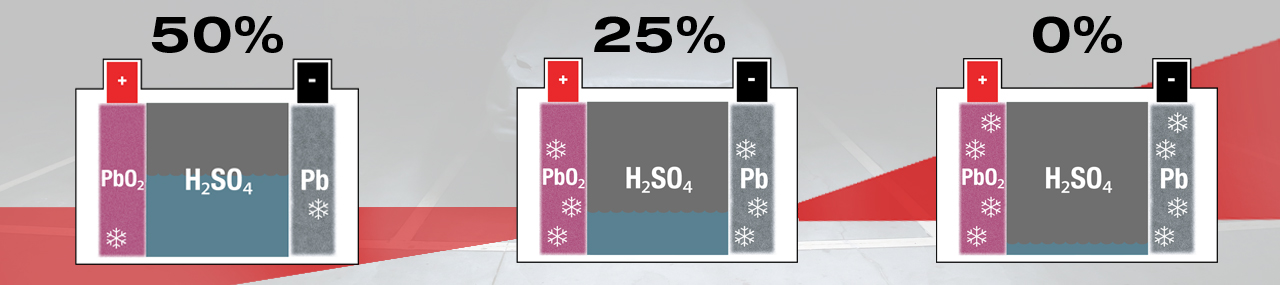
Within a lead-acid battery the main chemical components are lead (Pb) cathode plates, lead-oxide (PbO2) anode plates and electrolyte that is diluted sulphuric acid (H2SO4).
Electricity is created within a lead-acid battery when the chemicals react with each other, but as a result new 'waste' chemicals are created. During discharge, sulphur (SO4) from the electrolyte fuse with lead (Pb) on the surface of the plates, creating lead-sulphate (PbSO4). A lead-acid battery is completely discharged when all sulphate (SO4) has been removed from the electrolyte, leaving mostly water (H2O), and all plate surfaces are covered with lead-sulphate. When recharged the lead-sulphate atoms are dismantled and recycled, lead (Pb) remains at the plates and the sulphate (SO4) is re-absorbed into the electrolyte, once again creating sulphuric acid (H2SO4). A lead-acid battery is fully charged when no lead-sulphate (PbSO4) remains.
In an active battery the presence of lead-sulphate (PbSO4) poses no danger as it is continuously created and dismantled. When the battery is left in a partially discharged state for too long the unconverted lead-sulphate (PbSO4) crystalizes and hardens on the surface of the plates, making it more difficult to dismantle when the battery once again receives charge. The reduced lead surface area affects the battery’s ability to deliver and hold power i.e. the battery has become smaller (less Ah capacity) and therefore weaker. Maintaining a lead-acid battery at full charge will avoid loss of power. Alternatively recharge frequently, ideally before the battery drops below 75% S.O.C..
The battery’s electrolyte is diluted sulphuric acid (H2SO4 + H2O).
Within a charged battery the concentration of sulphuric acid (H2SO4) is higher than water (H2O), which decreases the electrolyte’s freezing point to -95°F / -71°C.
As the battery discharges the concentration of sulphuric acid reduces and water content increases, which increases the freezing point of the electrolyte towards that of water. A battery at 50% charge level has a freezing point of approximately -22°F / -30°C, but a completely discharged battery’s electrolyte is mostly water (H2O) with a freezing point of 32°F / 0°C.
When water freezes it expands by 120% and will break the battery from within.
Maintaining a lead-acid battery at full charge prevents the battery from freezing even in extreme cold.
An excess of lead-sulphate within the battery prevents electricity flow at normal charging voltages (e.g. 14.4V for a 12V battery) – the battery is unable to hold a charge. To recover the battery a higher voltage is required, to overcome the resistance caused by too high levels of lead-sulphate and force electricity to flow. OptiMate Battery Saving chargers for lead-acid batteries can recover dead flat batteries from as low as 0.5V.
Once all lead-sulphate has been converted, continued electricity flow causes the electrolyte to increase in temperature and emit hydrogen and oxygen. The electrolyte will eventually ‘dry out’, causing the battery to demand even more charge current that will destroy the battery’s plates. If trapped, hydrogen and oxygen can cause an explosion.
A Lithium LFP battery has three main components in each cell, the cathode made of Lithium Ferrous Phosphate (LFP - LiFePo4), anode made of Carbon (C ) and the Lithium based electrolyte (LiCIO4 - Lithium perchloride) that transfer lithium-ions between the the anode and cathode.
Lithium batteries create electricity by moving Lithium Ions (Li-Ions) between the cathode and the anode through the electrolyte. No additional waste chemical is created.
When discharged (battery delivering energy) Li-Ions move from the anode to the cathode. When recharged the Li-Ions move from cathode back to the anode.
A lithium battery remains in a good State Of Health if there is always sufficient Li-Ions at the cathode, anode and within the electrolyte i.e. the battery is never discharged too low and never charged too high.
All the lithium-Ions are concentrated at the cathode with very little at the anode. This causes the carbon anode to lose structure and it cannot receive lithium-ions at normal charge rate. If recharged too fast, or even ‘jump started’, lithium ions bombard the carbon anode and causes it to overheat. To recover the battery a very slow controlled charge at low current is required, to allow the anode to slowly absorb lithium-ions until it can once again receive charge normally. OptiMate Lithium chargers for LFP Lithium batteries can recover dead flat batteries from as low as 0.5V.
When fully charged the carbon anode of each cell is fully packed with lithium ions and cannot receive any more, but continued electricity flow forces lithium-ions to try entering the anode, causing the carbon to overheat. Once it reaches a critical temperature, it will self-combust, and the battery may catch fire.